FILE PHOTO: AstraZeneca’s logo is reflected in a drop on a syringe needle in this illustration taken November 9, 2020. REUTERS/Dado Ruvic/Illustration/File Photo
The global need to exponentially increase the number of people immunized with vaccines of various types, technologies and sources becomes not only an obsession for scientists and laboratories – who do not cease to articulate more and more alliances and hub biotech to optimize their production – but also for governments.
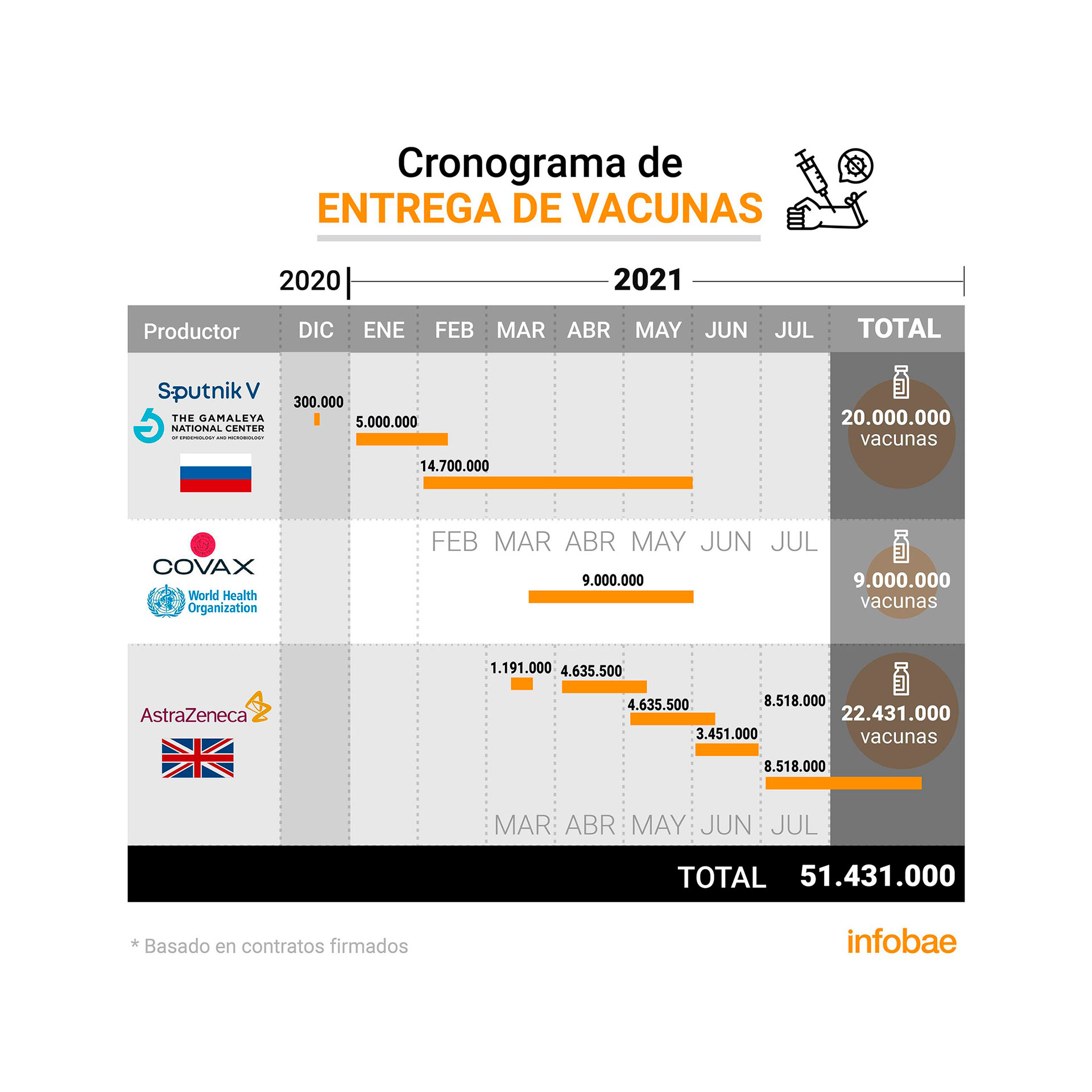
In this context, and as Infobae learned, last week a confidential meeting was held between the authorities of the Ministry of Health of the Nation and the AstraZeneca laboratory where an additional 1.2 million doses were managed in advance from February 2021, which will be added to the 22.4 million that the Government has already signed by contract with the Anglo-Swedish laboratory in joint production with the local biotechnology company mAbxience and the Mexican Liomont; and outside the 2.2 million vaccines that the COVAX agency promised the country.
The arrival of these new added doses that come from the global supply network of the Oxford-AstraZenecaAZ binomial are contemplated under the following scheme: 580 thousand will arrive in February 2021 and the other 580 thousand in March 2021.
Today was a day of announcements for the AstraZeneca laboratory that struggles not to lose its leading place in the race and provision of vaccines to the world, and above all having been the first scientific node that started with the research and the search to find a vaccine safe and effective against COVID-19.
The main analysis of Phase III clinical trials from the UK, Brazil and South Africa, published as preprint in The Lancet, confirmed that the AstraZeneca vaccine against COVID-19 is safe and effective in preventing COVID-19, with no severe cases or hospitalizations , more than 22 days after the first dose.
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/D63MMNPPCNSCDEVPO3YMYM4UX4.jpg) The Oxford-AstraZeneca vaccine demonstrated in scientific papers a 100% reduction in hospitalization in cases of COVID-19. And it revealed 76% efficacy with the first dose and 82% with the second at 3 months. REUTERS / Dado Ruvic / Ilustration
The Oxford-AstraZeneca vaccine demonstrated in scientific papers a 100% reduction in hospitalization in cases of COVID-19. And it revealed 76% efficacy with the first dose and 82% with the second at 3 months. REUTERS / Dado Ruvic / Ilustration
The results demonstrated a vaccine efficacy of 76% (CI: 59% to 86%) after a first dose, with protection maintained until the second dose. With an interval between doses of 12 weeks or more, the efficacy of the vaccine increased to 82% (CI: 63%, 92%). This is the first information of the brake data in virus transmission.
The analysis also showed the vaccine’s potential to reduce asymptomatic transmission of the virus, based on weekly swabs obtained from volunteers in the UK trial. The data showed that positive CRP readings were reduced by 67% (CI: 49%, 78%) after a single dose and by 50% (CI: 38% to 59%) after the two-dose regimen, supporting a substantial impact on transmission. of the virus.
The main efficacy analysis was based on 17,177 participants accumulating 332 symptomatic cases from phase III trials in the UK (COV002), Brazil (COV003) and South Africa (COV005) led by the University of Oxford and AstraZeneca, 201 more cases than those previously reported.
Controlling the transmission of the virus becomes a priority because the more it “runs” between people, the more likely it is to mutate. That is why access to vaccines is essential to pierce the pandemic for the new coronavirus.
The type of technology that this AstraZeneca COVID-19 (ex AZD1222) vaccine has is based on chimpanzee adenovirus and its logic of origin production and application of the doses makes it an accessible development under the concept Non profit that from the beginning of the pandemic the Oxford researchers raised around the scientific finding. The production from the beginning was also raised through the different associations with biotechnological hubs, such as the Argentine mAbxcience (from the Insud Group) and the Mexican laboratory Liomont; and in association with Grupo Slim to supply the region.
The technological hub that allows the Anglo-Swedish laboratory to scale its production in the renowned Serum Institute of India, which produces the Oxford vaccine, and which promised to maintain a value similar to that of the first contract signed directly with AstraZeneca. That is: $ 4 per dose, at a rate of US $ 8 for the complete immunization.
These additional doses that will arrive in the coming weeks to Argentina maintain the consideration that the Oxford-AstraZeneca laboratory received as it is the second country in the world – behind the United Kingdom and the approval it provided through its regulatory body MHRA – in approving the emergency use of the vaccine through the National Administration of Medicines, Food and Medical Technology (ANMAT).
I KEEP READING:
Oxford-AstraZeneca vaccine demonstrates 100% reduction in hospitalization in COVID-19 cases
In Europe they analyze reserving the Oxford-AstraZeneca vaccine for those under 65
The advantages of the Oxford vaccine that could change the course of the pandemic in the region











